P07-23
A small molecule inhibitor that binds to the unstable state of its target kinase DYRK1A demonstrates slowly dissociation from the complex
Sora SUZUKI *1, Koji UMEZAWA2, Gaku FURUIE1, Daichi NAKAMURA3, Ninako KIMURA1, Masato YAMAKAWA1, Yuto SUMIDA3, 4, Takashi NIWA3, 4, 5, Takamitsu HOSOYA3, 4, Kii ISAO1, 2
1Department of Agriculture, Graduate School of Science and Technology, Shinshu University
2Institute for Biomedical Sciences, Shinshu University
3Laboratory for Chemical Biology, RIKEN BDR
4Laboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University
5Graduate School of Pharmaceutical Sciences, Kyusyu University
( * E-mail: 23as104a@shinshu-u.ac.jp )
In small molecule drug discovery, inhibitors that dissociate slowly from their target proteins have been being highly sought. The inhibitory effect of such inhibitors is maintained even when their concentration in the body decreases due to metabolism and excretion. As a result, the frequency of administration can be reduced, thereby alleviating side effects.
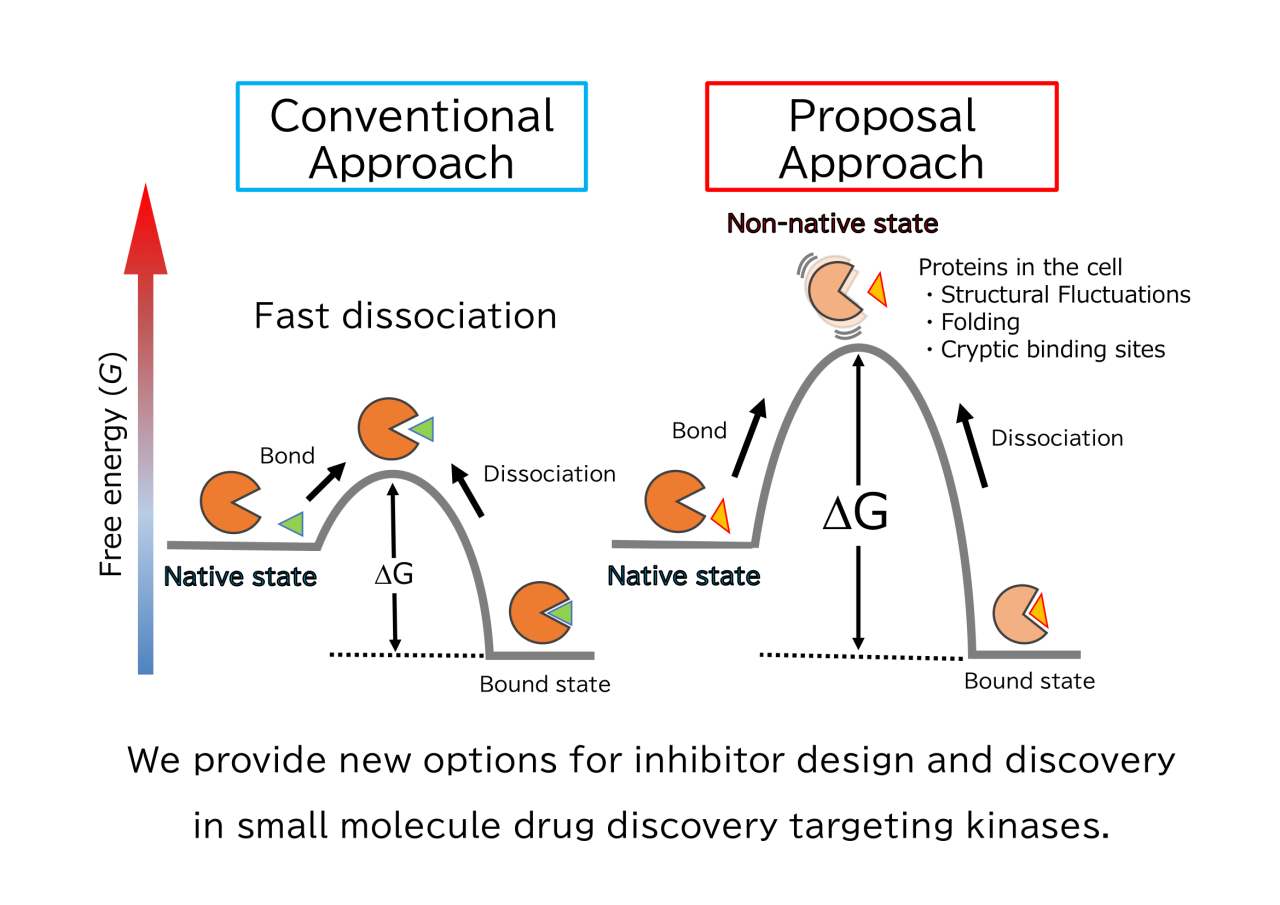
In this presentation, we report that small molecule inhibitors that selectively target the non-native states of proteins dissociate slowly from their complexes. The native state is the stable conformation of the protein, characterized by the lowest free energy. In contrast, the non-native state is an unstable conformation with a partially denatured protein structure and higher free energy. Therefore, when small molecule inhibitors selectively bind to the non-native state, the free energy significantly decreases compared to binding to the native state. Generally, for a small molecule inhibitor to dissociate from the complex, the complex must obtain the lowered free energy from the surrounding environment. This implies that a significant amount of free energy is required from the environment for the inhibitor to dissociate from the complex. However, since the frequency of the complex obtaining such a large amount of free energy from the environment is low, dissociation inevitably becomes slow.
We validated this theory using the kinase DYRK1A, which is involved in neurological disorders, and a selective small molecule inhibitor for its non-native state, FINDY. Experimental results showed that FINDY did not dissociate from its complex with DYRK1A. Furthermore, we analyzed the binding structures of an inhibitor for the native state of DYRK1A and a structural analogue that is selective for the non-native state. By comparing these two binding structures, we successfully identified the structural characteristics of inhibitors that exhibit slow dissociation. This study provides new options for inhibitor design and discovery in small molecule drug discovery targeting kinases.