P07-11
Structure and Interaction Analysis of Nucleic Acid Encapsulated ssPalm Lipid Nanoparticles by Multiscale Simulation.
Naoko KONAMI *1, Koji OKUWAKI2, Hiroki TANAKA3, Hidetaka AKITA4, Kenjirou HIGASHI4, Takayuki FURUISHI5, Etsuo YONEMOCHI6, Kaori FUKUZAWA1
1Graduate School of Pharmaceutical Sciences, Osaka University
2JSOL Corporation
3Graduate School of Pharmaceutical Sciences, Tohoku University
4Graduate School of Pharmaceutical Sciences, Chiba University
5Juntendo University Faculty of Pharmacy
6School of Pharmacy at Narita, International University of Health and welfare
( * E-mail: konami-n@phs.osaka-u.ac.jp )
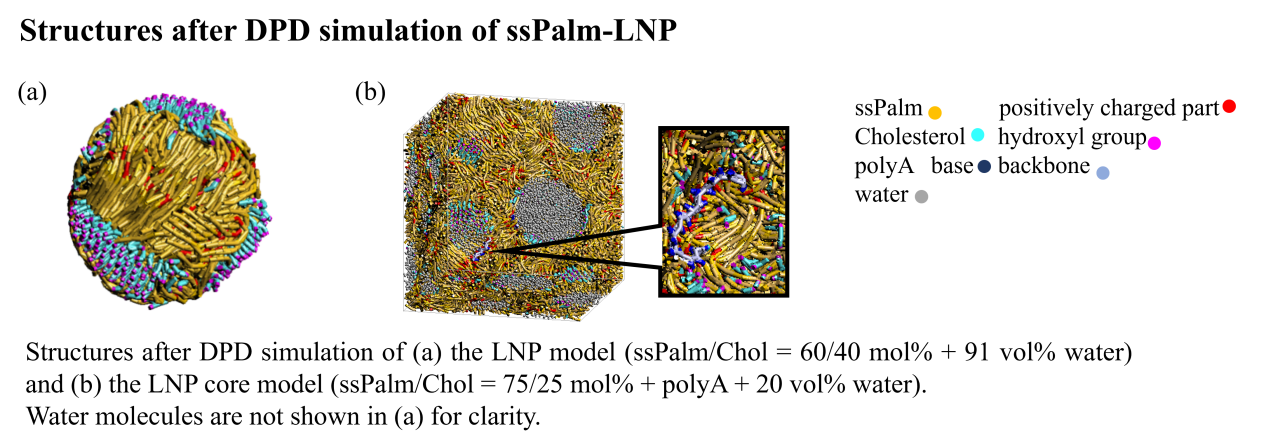
ssPalm is an SS-cleavable and pH-activated lipid-like material for nucleic acid delivery. Depending on the linker structure of oleic acid scaffold and tertiary amine, the transfection efficiency increases in the order of ssPalmO-Ph (SSOP), ssPalmO-Bn (SSOB), and ssPalmO-P4C2 (SSOC). In this study, we analyzed the structure of ssPalm-LNPs and the interaction between lipids by molecular simulation in order to understand the influence of lipid linker structure and composition on LNP structure. The molecular structures of ssPalm, cholesterol (Chol), and polyA were divided into coarse-grained particles, and the interaction parameters for the Dissipative Particle Dynamics (DPD) method were calculated using the Fragment Molecular Orbital (FMO) method. Two models were created using the FMO-DPD method with the COGNAC program: (a) LNP models and (b) LNP core models, to evaluate particle structure in water and the structure of encapsulated nucleic acids within the LNP, respectively. The LNP model comprised ssPalm/Chol at a 60/40 molar ratio with 91 vol% water, while the core model contained ssPalm/Chol at a 75/25 molar ratio along with polyA and 20 vol% water. Both models were simulated for 1 million steps within a 100,000-particle simulation box. Then, all -atom models were constructed by the reverse mapping approach based on the DPD structure. Molecular Dynamics (MD) calculations were performed for 100 ns using the Amber10:EHT force field in the AMBER program. Finally, FMO calculations of about 30,000 atoms were conducted for the MD structure after 100 ns at the MP2/6-31G level using the ABINIT-MP program. In all three ssPalm-LNP structures, Chol was not miscible with ssPalm, and clusters of Chol were formed on the LNP surface. The hydroxyl groups of Chol faced the outer aqueous phase of the particles. FMO calculations show that SSOP and SSOB with benzene rings have energetically more stable interactions between ssPalm compared to SSOC without benzene rings. It was suggested that the stabilization of LNPs by introducing benzene rings is involved in enhancing transfection efficiency. In addition, an endo-aqueous phase was formed in the LNP core, and Chol clusters were also observed at an interface between lipid and water. PolyA was presented at the interface, surrounded by charged portions of ssPalm, while avoiding Chol clusters. Multi-scale simulations combining DPD, MD, and FMO enable the structure and inter molecular interaction of LNPs at the atomic level, providing insights for enhancing their activity.