P06-03
Single-Cell Transcriptome Analysis Reveals Roles of GABA Receptors in the Connectivity of Dorsal-Ventral Motor Neurons in C. elegans
Xingran WANG *, Kosuke HASHIMOTO, Kenji MIZUGUCHI
Laboratory for Computational Biology, Institute for Protein Research, Osaka University
( * E-mail: u323248e@ecs.osaka-u.ac.jp )
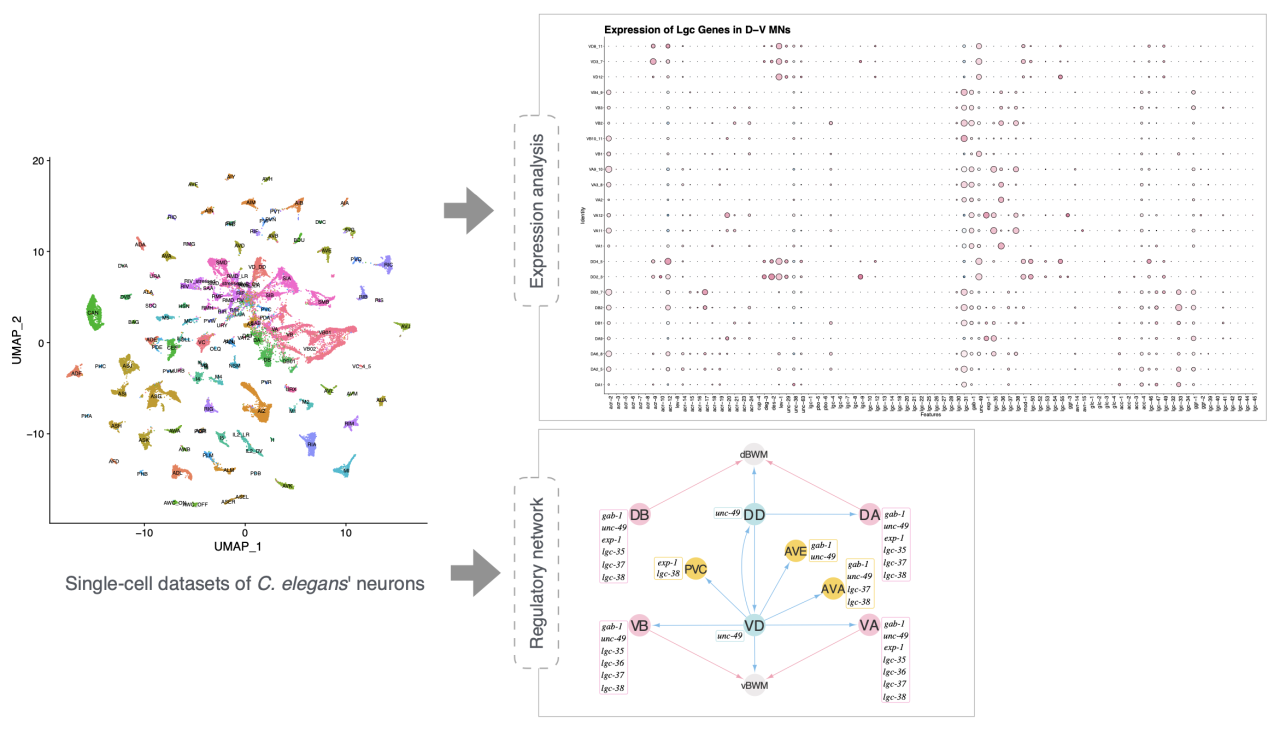
The cys-loop receptors, encoded by lgc genes, play a pivotal role in chemical neurotransmission and are targets for drugs treating neurological disorders. While mammals possess approximately 45 lgc genes, C. elegans with a simple nervous system has 102 lgc genes. Many of these genes are absent in the human genome, posing intriguing questions about their functions. Among these 102 lgc genes, only about 20% have been characterized to date, leaving the functions of the majority yet to be elucidated.
In this study, we employed single-cell transcriptome analysis to delineate the expression patterns of cys-loop receptors within C. elegans neurons. The dataset encompasses 70,296 neurons from L4 stage larvae, representing all 118 canonical neuron classes. Using R Seurat package, we discovered that lgc genes are primariy expressed in motor neurons, with particularly high levels in dorsal-ventral motor neurons (D-V MNs). Notably, D-V MNs show elevated expression of the seven GABA receptors identified to date in C. elegans, which include both inhibitory and excitatory subtypes. Each D-V MN type demonstrates distinct expression profiles among these GABA receptor subtypes. Moreover, both excitatory and inhibitory GABA receptors can be co-expressed on the same D-V MN, adding complexity to their roles in locomotion beyond traditional neuron-muscle junctions. By integrating the expression and the connectivity of D-V MNs, we found that mixed-type GABA receptors enable interactions with defecation interneurons (AVL and DVB). These interneurons regulate the defecation motor program by modulating D-V MNs, which in turn affects body wall and intestinal muscles. This study highlights the intricate roles of GABA receptors in neural connectivity and lays a robust foundation for future research to elucidate the underlying mechanisms of GABAergic regulation in motor behaviors.