P03-10
SAR analysis and visualization utilizing a fragment-based approach: Application to a public data analysis of Targeted Protein Degrader
Hiroyuki HAKAMATA *, Kosuke TAKEUCHI, Toshiaki WATANABE
Modality Research Laboratories l, R&D Division, Daiichi Sankyo Co., Ltd.
( * E-mail: hiroyuki.hakamata@daiichisankyo.com )
Structure-Activity Relationship (SAR) represents chemical landscape between chemical structures and their biological activities, which plays a central role in medicinal chemistry. Visualization of various information such as potency, ADMET and in vivo experiment data often provides medicinal chemists with new insights. In small molecule drug discovery, several visualization techniques such as R-group decomposition and followed by network analysis have been established to date [1]. Using these techniques effectively enables medicinal chemists to come up with next compound designs to be synthesized, which can lead to an acceleration in lead optimization campaign.
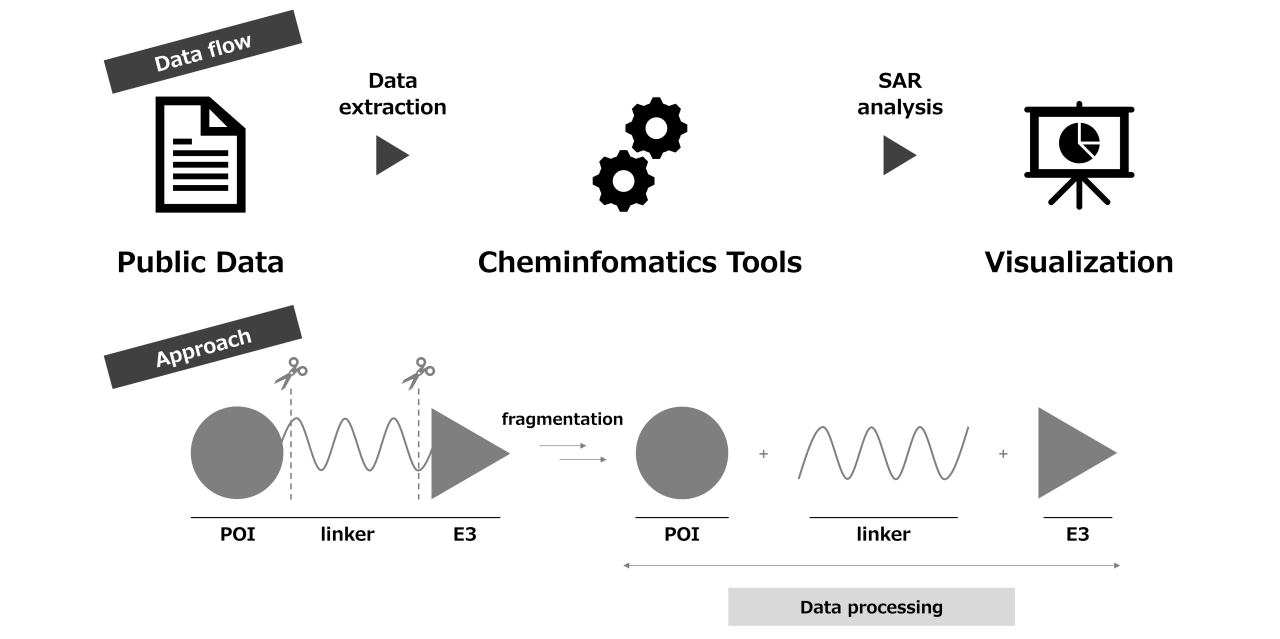
Targeted Protein Degrader (TPD) is a middle-sized molecule composed of three fragments: Protein of Interest (POI) ligand, linker, and E3 ligand. With its degradation MOA instead of inhibition demonstrated by traditional small molecules, it is highly expected to be one of promising modalities. However, due to generally beyond rule of 5 chemical-space, there are numerous options on chemical modification to aim at sweat spot between activity and ADMET property. Besides, its unique MOA makes comprehensive data analysis complicated.
Since starting the examination of "Data-Driven Drug Discovery" (D4) in 2018, we have been leveraging cheminformatics methods such as the development of SAR tables and extracting information from public data, leading to improvement of operational efficiency in multiple drug discovery projects [2].
In this presentation, we will introduce the SAR analysis method using public data on TPD as a case study and discuss workflow for data extraction, molecular fragmentation, assigning physicochemical property values, and visualization.
References
[1] Dagmar Stumpfe, Jürgen Bajorath, Recent developments in SAR visualization, Med. Chem. Commun., 2016, 7, 1045-1055.
[2] Ryo Kunimoto, Jürgen Bajorath, Kazumasa Aoki, From traditional to data-driven medicinal chemistry: A case study, Drug Discovery Today, 2022, 27, 2065-2070.