P01-18
Mechanisms of Type-51 R-body conformational changes revealed by in silico methods
Hiroaki OHEDA *1, Toru EKIMOTO1, Tsutomu YAMANE2, Kosuke KIKUCHI3, Koki DATE3, Takafumi UENO3, Mitsunori IKEGUCHI1, 2
1Yokohama City Univ.
2RIKEN
3Tokyo Institute of Technology
( * E-mail: w235411b@yokohama-cu.ac.jp )
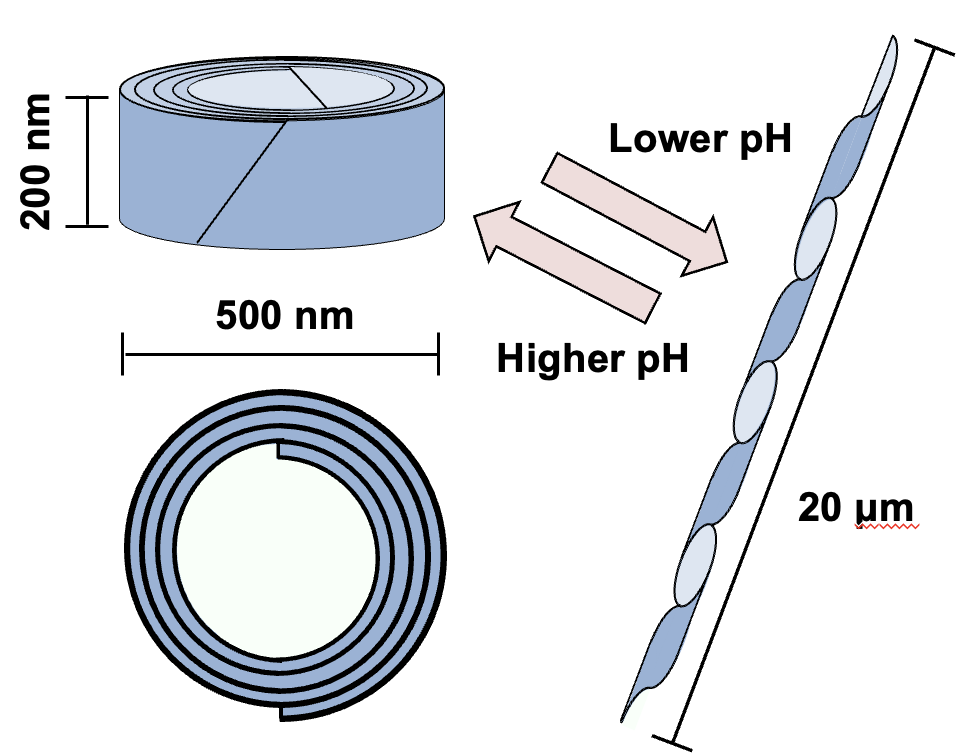
Type 51 Refractile Body (R-body) is a cylindrical and coiled protein polymer found in the cytoplasm of Caedibacter taeniospiralis, an intracellular symbiotic bacterium residing in Paramecium. The R-body is primarily composed of two major proteins, RebA and RebB. This polymer exhibits reversible structural changes: it can switch from a cylindrical form, approximately 200 nm in height, to a needle-like structure extending up to 20 μm in length, depending on the pH.
Recent studies utilizing solid-state NMR (SS-NMR) have revealed that RebA and RebB, the main components of the R-body, are predominantly composed of α-helices. These studies also identified several regions in the proteins that act as helix breakers. Despite these advancements in understanding its partial secondary structure, the unique coiled arrangement of the polymer significantly complicates the comprehensive analysis of its structure and function.
The goal of this study is to clarify the polymerization mechanisms and pH-dependent conformational changes of the R-body through various in silico methods. Molecular dynamics (MD) simulations were conducted based on a monomer structure predicted by AlphaFold2, which confirmed the presence of kinks at several residues. These kinked regions correspond to the helix breaker regions identified in previous SS-NMR studies. Furthermore, MD simulations under different pH conditions highlighted several key residues likely involved in the pH-dependent structural transitions of the R-body.
Future research will focus on constructing a comprehensive model that integrates these monomeric findings with experimental data. This will be followed by MD simulations aimed at further elucidating the mechanisms behind the dynamic structural transformations of the entire R-body.