P01-16
Conformational study of macrocyclic peptides in solvent by MD simulations to improve their membrane permeability
Ekishin YANAGI *1, Patricia BRANDL2, Stephanie M. LINKER2, Sereina RINIKER2, Takayuki KATOH1, R. H. P. van NEER 3, Hiroaki SUGA1
1Department of Chemistry, School of Science, The University of Tokyo
2Computational Chemistry, Institute of Molecular Physical Science, Department of Chemistry and Applied Biosciences, ETH Zurich
3National Center for Advancing Translational Sciences, National Institutes of Health
( * E-mail: ekishin@g.ecc.u-tokyo.ac.jp )
Short macrocyclic peptides, composed of 8 to 15 amino acids, are attracting attention as protein-protein interaction (PPI) inhibitors. Their small and constrained structure allows for both a unique combination of conformational rigidity and flexibility. This enables peptides to fit easily into flat and large surface areas of target proteins, resulting in selective and strong interactions. However, membrane permeability decreases as their molecular size increases, which has been the major bottleneck for therapeutic peptide development targeting intracellular proteins.
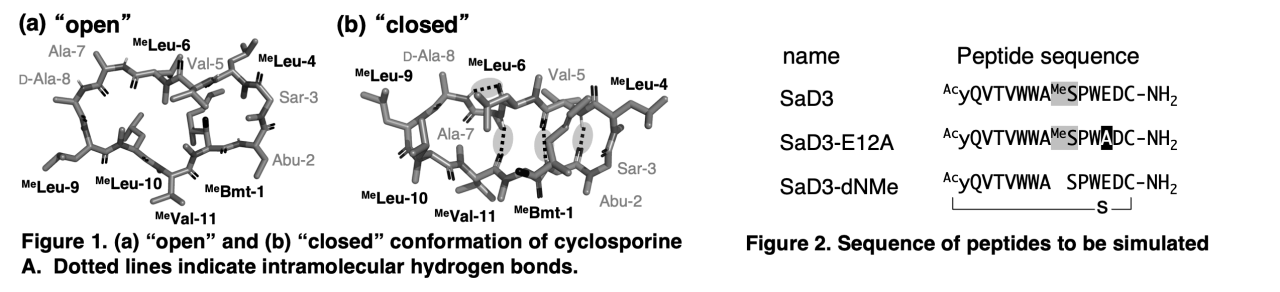
Despite many peptides not being membrane-permeable, there are some notable exceptions, such as cyclosporine A (CsA), known as possessing high membrane permeability. Membrane-permeable peptides are said to have the following characteristics.
1. Having few charged amino acids, since the surface of the membrane is hydrophobic.
2. Including N-methyl amino acids; CsA also contains many N-methyl amino acids.
3. Having conformational flexibility between “open” (no intramolecular hydrogen bonds) and “closed” (intramolecular hydrogen bonds are formed and polar groups are maximum shielded); computational studies have demonstrated that CsA has this feature (Figure 1).
As the conditions for membrane-permeable peptides are still not fully understood, conformational analysis by Molecular dynamics (MD) simulations is crucial to our further understanding.
The aim of this research is to establish a generic methodology to improve the membrane permeability of bioactive macrocyclic peptides with poor membrane permeability by combining wet and dry experiments. To achieve this purpose, we chose SaD3, which showed strong binding affinity and inhibitory activity against Staphylococcus aureus co-factor-independent phosphoglycerate mutase (Sa-iPGM), as a starting point (Neer et al. ACS Chem. Biol. 2022, 17). SaD3 has two charged amino acids and one N-methyl amino acid and is not membrane-permeable. As Sa-iPGM is an intracellular target, improving the membrane permeability of SaD3 could make SaD3 a powerful drug for infectious diseases.
To this end, we ascertained whether SaD3 shows conformational flexibility between “open” and “closed”. Also, we investigated how conformation would change when the charged and N-methyl amino acids were replaced by other amino acids. MD simulations in two solvents were performed for SaD3, SaD3-E12A (Glu at position 12 was changed to Ala), and SaD3-dNMe (MeSer at position 9 was changed to Ser) (Figure 2). The results showed that the SaD3 tend to take both “open” and “closed” conformation. Additionally, we found that the substitution of even a single amino acid could significantly change the conformation of the peptides.