P01-10
Epicatechin n-mers (n ≥ 5) adopt more compact conformations than catechin n-mers
Toshiaki UEDA *1, KAWAHARA TAKESHI1, 2, MAKABE HIDEFUMI1, 2, UMEZAWA KOJI1, 2
1Graduate School of Science and Technology, Shinshu University
2Institute for Biomedical Sciences, Shinshu University
( * E-mail: 24bs104a@shinshu-u.ac.jp )
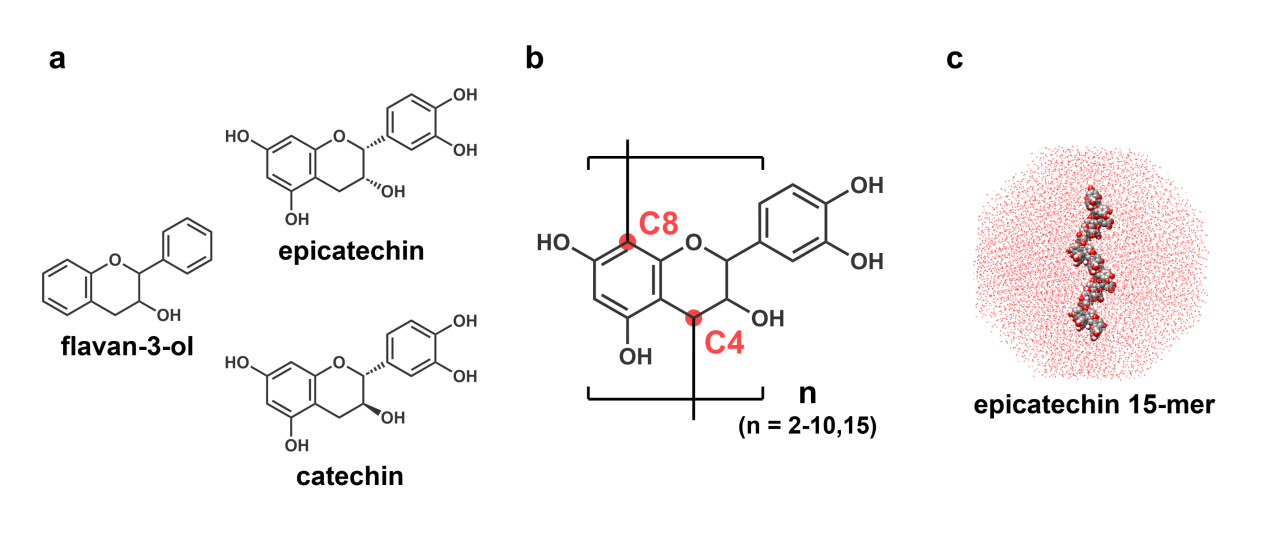
Epicatechin and catechin belong to flavan-3-ol of flavonoids. Epicatechin is a cis-trans isomer of catechin (Fig. a). These oligomers (n-mers, Fig. b) are connected by the inter-flavan (C4-C8) bonds and are found in natural products. The long epicatechin n-mers (n ≥ 5) have an anti-invasive activity against cancer cells. In contrast, the long catechin n-mers (n ≥ 5) do not. It suggested that the conformations of the long epicatechin and catechin n-mers are different.
The purpose of our study was to characterize conformational properties of epicatechin and catechin n-mers. The conformational ensembles of epicatechin and catechin n-mers (n = 2-10, and 15) were calculated with an all-atom model in explicit solvents (Fig. c) by an enhanced-sampling method, multicanonical molecular dynamics simulation. The 300K conformational ensembles were analyzed.
The end-to-end distances were calculated to understand the molecular lengths of epicatechin and catechin n-mers. The results showed that the lengths of the long epicatechin n-mers were shorter than those of the long catechin n-mers. The residue-residue contacts and solvent-accessible-surface area per residue were analyzed to find intramolecular interaction. In the long n-mers (n ≥ 5), epicatechin residues made the contacts with higher probability than catechin, and epicatechin residues were buried inside the molecule. These results showed that intramolecular interactions of epicatechin residues may lead to the compact structure in the long epicatechin n-mers compared to catechin.